Global Health Colloquium - Guest Speaker
David H. Sherman, PhD
Hans W. Vahlteich Professor of Medicinal Chemistry
University of Michigan Life Sciences Institute
“The Discovery and Development of a New Broad-Spectrum Polyketide Antibiotic; and Structure and Rearrangements of a Complete Polyketide Synthase Module During its Catalytic Cycle”
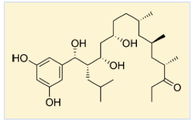
Siderophores are high-affinity iron chelators produced by microorganisms and frequently contribute to the virulence of human pathogens. Targeted inhibition of the biosynthesis of siderophores staphyloferrin B of Staphylococcus aureus and petrobactin of Bacillus anthracis hold considerable potential as a single or combined treatment for methicillin-resistant S. aureus (MRSA) and anthrax infection, respectively. The biosynthetic pathways for both siderophores involve a nonribosomal peptide synthetase independent siderophore (NIS) synthetase, including SbnE in staphyloferrin B and AsbA in petrobactin. We developed a biochemical assay specific for NIS synthetases to screen for inhibitors of SbnE and AsbA against a library of marine microbial-derived natural product extracts (NPEs). Analysis of the NPE derived from Streptomyces tempisquensis led to the isolation of the novel antibiotics baulamycins A (BmcA) and B (BmcB). BmcA and BmcB, which are polyketide natural products from Costa Rican microbiota displayed in vitro activity with IC50 values of 4.8 μM and 19 μM against SbnE and 180 μM and 200 μM against AsbA, respectively. Kinetic analysis showed that the compounds function as reversible competitive enzyme inhibitors. Liquid culture studies with S. aureus, B. anthracis, E. coli and several other bacterial pathogens demonstrated the capacity of these natural products to penetrate the cell wall and inhibit growth of both Gram- positive and Gram-negative species. These studies provide proof-of- concept that natural product inhibitors targeting siderophore virulence factors can provide access to novel broad-spectrum antibiotics, which may serve as important leads for the development of potent anti-infective agents.
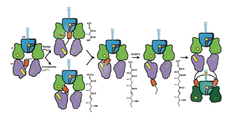
Polyketide natural products constitute a broad class of compounds with diverse structural features and biological activities. Their biosynthetic machinery, represented by type I polyketide synthases, has an architecture in which successive modules catalyze two-carbon linear extensions and keto group processing reactions on intermediates covalently tethered to carrier domains. We determined sub-nanometer resolution electron cryo-microscopy (cryo-EM) structures of a full-length PKS module in three key biochemical states of its catalytic cycle. Each biochemical state was confirmed by bottom-up liquid chromatography Fourier transform ion cyclotron resonance mass spectrometry (LC/FT-ICR MS). The ACP domain is differentially and precisely positioned after polyketide chain substrate loading on the active site of KS, after extended β-keto- intermediate formation, and after β- hydroxy product generation. The structures reveal the ACP dynamics for sequential binding to catalytic domains within the reaction chamber, and how the ACP positions the elongated and processed polyketide substrate for transfer to the next module in the PKS pathway. During the enzymatic cycle the KR domain undergoes dramatic conformational rearrangements that enable optimal positioning for reductive processing of the ACP-bound polyketide chain elongation intermediate. These findings have crucial implications for the design of functional PKS modules and for the engineering of pharmacologically relevant PKS pathways.